what types of plants are important to the tropical andes
Introduction
Andean Variety Gradients
The northern and fundamental Andes accept been identified every bit globally outstanding centers of plant diversity across life forms and phylogenetic groups (Gentry, 1982; Myers et al., 2000; Barthlott et al., 2005; Mutke et al., 2011). Andean biodiversity has primarily latitudinal and altitudinal dimensions. There have been several studies addressing altitudinal patterns of diversity in the Andes. For birds, a widely cited study by Terborgh (1977) shows a monotonic decrease of bird species richness with elevation in the Peruvian Andes. In the Peruvian flora, epiphytic orchids bear witness highest species richness at lower and mid elevations up to two,000 g above ocean level (g a.s.50.). Terrestrial orchid diversity peaks effectually 2,000–2,500 m a.s.l. and overall species richness of orchids peaks at ca. 1,500–2,000 m a.southward.l. (Ibisch et al., 1996). Similar patterns were documented by Küper et al. (2004) for Ecuadorian epiphytic plants. In dissimilarity, overall diverseness of tree taxa and of the entire flora across taxonomic groups and life forms peaks at low elevations (<500 m a.s.fifty.) both in Peru and Ecuador (Braun et al., 2002; Jørgensen et al., 2011; Mutke, 2011) – showing parallel trends to the bachelor expanse per elevational zone. Plant endemism in Peru peaks at i,500–3,000 m across life forms, a effigy based on the overall predominance of epiphytes, terrestrial herbs, and shrubs amid endemic taxa (Van Der Werff and Consiglio, 2004). "Density" of endemic species (species per 1,000 kmtwo) in their study was fifty-fifty found to be more than than ten times college at ii,000–3,500 m than in the Amazonian lowlands (0–500 m, Van Der Werff and Consiglio, 2004) – yet, this was computed based on a linear species-surface area relationship, which is at least problematic. Variety of predominantly tropical, mostly shrubby families (Acanthaceae, Araceae, Melastomataceae, and Palmae) in Bolivia peaks at relatively lower elevations (Kessler, 2001, 2002). Similar results accept been found by other authors and overreaching patterns for the Andes, based on the most up-to-engagement taxonomic data available, support this conclusion (Jørgensen et al., 2011). Topographical and habitat heterogeneity together with the water and free energy balance are considered as the overall nearly prominent factors influencing levels of plant diverseness at the geographical scale (Mutke et al., 2001; Braun et al., 2002; Kreft and Jetz, 2007; Jørgensen et al., 2011), but their detailed interplay in the Andes has then far eluded analysis.
Evolution of Tropical Andean Taxa
Several recent studies attempt to identify the evolutionary processes that are at the basis for the diversity gradients observed. Gentry (1982) already postulated an "explosive speciation and adaptive radiations" in shrubs and epiphytes as a direct consequence of Andean uplift, especially in the northern Andes. It has been hypothesized that Andean uplift was the causal agent for increased diversification in the shrub-genus Hedyosmum, a genus of some 44 species in the Chloranthaceae (Antonelli and Sanmartín, 2011). There, it is assumed that uplift itself separated populations and provided the ground for allopatric speciation, since diversification in Hedyosmum evidently took identify in parallel to Andean uplift. Similarly, diversification patterns in Rubiaceae are hypothesized to correlate closely to paleogeography in the Andes and the Amazon, with a long-lasting separation of the northern and the central Andes (Antonelli et al., 2009). Similar arguments have been brought forward for Andean Macrocarpaea (Struwe et al., 2009). Conversely, several publications show that the diversification of particular high Andean clades predates the formation of the current habitats (Bell and Donoghue, 2005; Hershkovitz et al., 2006; Palazzesi et al., 2009, 2012; Emadzade et al., 2010). Some other, by now widely documented, phenomenon is the very recent and explosive radiation of high Andean (Páramo and Puna) groups. It appears to take essentially taken place after the main Andean uplift (e.g., Gentianella and Halenia, Hypericum, Lupinus, Valeriana: von Hagen and Kadereit, 2001; Kadereit and von Hagen, 2003; Bong and Donoghue, 2005; Hughes and Eastwood, 2006; Nürk et al., 2013). Overall, a wide range of high Andean (Páramo and Puna) taxa seem to have radiated extensively and very recently (Madriñán et al., 2013). These examples might bespeak that mid-pinnacle diversity predates high-height multifariousness and has a strong historical component. However, upper slope taxa, from elevations of ii,000–3,500 thou, have not been extensively studied so far and the phylogenetic studies do not include an analysis of the detailed altitudinal and latitudinal diversity patterns of the plant groups concerned. In this altitudinal band we would await to observe a potent historical signal in latitudinal variety, especially in high and mid-elevation found groups, since Andean uplift took place in dramatically different time periods, with the central Andes as the oldest part of the tropical Andes, followed past an uplift of the northern Andes and – last of all – an uplift of the connectedness of these two in the Amotape–Huancabamba Zone (AHZ; Hoorn et al., 2010). It is therefore surprising that two typical mid-slope groups of shrubby angiosperms, namely Macrocarpaea (Struwe et al., 2009) and Iochrominae (Solanaceae: Smith and Baum, 2006), announced to have centers of diverseness and possibly ancestral areas in this AHZ, which is at odds with geological history.
The Amotape–Huancabamba Zone – Phytogeographical Barrier or Singled-out Phytogeographical Region?
This region along the border of Ecuador and Peru is of particular interest, considering of many Andean establish groups with elevated levels of diversity and narrow endemicity. It has been variously termed northern Peruvian Depression, Huancabamba Deflection, Piura Split, and the Huancabamba Low, and is nowadays generally termed AHZ (Young and Reynel, 1997). This region, and most importantly the lowest part of the Andes in this region (lowest laissez passer at v.5° South) has frequently been referred to as a barrier for the dispersal of Andean plants (Vuilleumier, 1968; Molau, 1988; Prance, 1989), virtually recently by Richter et al. (2009). Berry (1982) was probably the showtime to argue against the theory of a phytogeographical bulwark and for the recognition of a distinct phytogeographical region in this part of the Andes. Evidence to support this latter statement has been brought frontwards by a series of publications based on distribution data of a small set of Andean plant groups (Weigend, 2002, 2004a; Weigend et al., 2005; Struwe et al., 2009). Some studies presenting biogeographical conclusions in support of a biogeographic barrier give no explicit source of distribution data at all (Bonaccorso, 2009; Chaves et al., 2011) and can be safely disregarded. Also, near studies on latitudinal diversity patterns in individual plant groups usually take pre-divers geographical units as the footing for a distributional analysis, i.eastward., either dividing the tropical Andes into two units (north and south of the Huancabamba deflection: Molau, 1988; Cosacov et al., 2009; Antonelli and Sanmartín, 2011) or three regions (northern Andes, AHZ, and central Andes: Weigend, 2002, 2004a; Weigend et al., 2005; Smith and Baum, 2006; Struwe et al., 2009). Even more than narrowly defined geographical units are followed in some botanical (Antonelli et al., 2009) and zoological (Weir, 2009) studies. In all these cases, the coding influences the patterns found, since fine-scale recognition of distribution limits is impossible when taxa are a priori assigned to geographical units. All taxa only found (somewhere) in one of the pre-divers units seem to underscore the presence of a biogeographical barrier betwixt the units, and this is the grossly erroneous interpretation that has more often than not been provided.
Data for the Present Article
There is frequently a strong collection bias in published distribution information and the main strong betoken of the nowadays study is that the distribution data from herbarium cloth have been extensively supplemented past field studies between 1993 and 2012, covering peculiarly the about poorly known parts of Peru (eastern slope, northern Peru), then that the underlying data are rather all-encompassing. Also, all plant determinations in our study are based on disquisitional taxonomical work (comparison of types, revisionary work), and then that underreporting is a relatively pocket-sized cistron and misidentifications are minimized. The written report groups appear to be particularly suited to a study of the diversification patterns in the Andes, since they are present throughout the tropical Andes, two reach into Patagonia in the S (Ribes, Urtica). They are also virtually mutual at intermediate elevations. Equally mentioned above, these are of particular involvement every bit far as diversification patterns are concerned and at the same time are the to the lowest degree understood region. All three groups are most various in disturbed, open habitats and secondary woods, relatively rare in main forests and thus represent an ecological group largely neglected in previous biogeographical studies. At elevations between 2,000 and iv,000 yard a.s.50. in the tropical Andes it is common to notice representatives of Ribes subg. Parilla sect. Andina, Nasa, and Urtica growing together, since they share overall similar requirements. To a bottom extent, this is also true for Urtica and Ribes subg. Parilla sect. Parilla in the southern Andes.
The Genus Nasa
Monophyletic Nasa belongs to the predominantly Neotropical plant family Loasaceae (Weigend, 2003). Currently, 128 taxa (98 species) are recognized, with a articulate center of diversity in northern Republic of peru (Weigend, 2003), where micro-allopatric subspecies and species are mutual (Dostert and Weigend, 1999; Henning and Weigend, 2009a). Nasa phylogeny is not fully resolved, but the group can be roughly subdivided into a range of breezy species groups, largely corresponding to growth habit and ecological preferences. Some of these have been confirmed by molecular data (Weigend et al., 2004; Weigend and Gottschling, 2006). Nasa shows a range of dissimilar growth forms, which more or less closely correspond to ecological preferences: annuals, biennials, and subperennials are from disturbed and/or highly seasonal habitats, deciduous shrubs are found on steep scree slopes, whereas the evergreen shrubs are mostly typical of the upper limits of the cloud forest (subpáramo habitats), rhizomatous perennials are largely restricted to high-Andean grasslands, whereas stoloniferous and lianescent taxa are found in secondary wood. Growth addiction is thus a good proxy for the vegetation type and dynamics that the species are found in (for details see Weigend and Rodríguez, 2003; Weigend et al., 2003; Weigend, 2004b; Henning and Weigend, 2009a; Henning et al., 2011).
The Genus Ribes Subgenus Parilla
Ribes is a largely north-temperate genus in monogeneric Grossulariaceae and is present in South America with ii different clades, both of which are exclusively berry-fruited (bird-dispersed), dioecious shrubs, classified in subgenus Parilla. Ribes subg. Parilla sect. Andina ( = Ribes sect. Andina) is largely restricted to the tropical Andes while Ribes subg. Parilla sect. Parilla ( = Ribes sect. Parilla) is restricted to the southern Andes (for details: Janczewski, 1907; Weigend et al., 2002; Weigend, 2007). The 2 sections are not closely related to each other and represent independent introductions from North America. Ribes sect. Andina contains 43 species overall (four of them undescribed) and extends from northern Argentina into Central America with a single species (Weigend and Binder, 2001a). The bulk of the species occur in the tropical Andes, where narrowly endemic species are common (Freire Fierro, 2004; Weigend et al., 2010). The shrubs are important components at the upper margins of the deject woods and in the subpáramo formation, with many species found in secondary forests and only a few of them in the undergrowth of primary forests. They are also plant in isolated patches at the base of rocks in Páramo and Puna vegetation. Ribes sect. Parilla comprises nine species overall (Weigend et al., 2008), and is restricted to Chile and Argentina, where it is mutual both in the forest undergrowth and at the upper limit of Patagonian forests.
The Genus Urtica
The genus Urtica (Urticaceae) is predominantly n-temperate in distribution, simply is represented with a total of 21 species in South America (Weddell, 1856, 1869; Weigend and Luebert, 2009). The members of Urtica are predominantly perennial, sometimes stoloniferous/rhizomatous, monoecious herbs from disturbed sites and secondary forest. Their one-seeded fruits are efficiently dispersed by animals and wind. South American taxa fall into two unrelated clades: Urtica gracilis s.fifty. with iv subspecies in the American Cordillera between the northern U.s.a. and Republic of chile and with one subspecies in temperate North America (Henning et al., 2014). U. gracilis southward.l. is here excluded from the analysis, as representing a minor, highly disjunct component. The grouping hither considered is monophyletic "American Urtica" (Farag et al., 2013; Henning et al., 2014) with a total of twenty species ranging from the eastern USA down to Argentina and Republic of chile, just with the majority (xiv species) restricted to Southward America. It is sis to a Macaronesian-Mediterranean clade (Farag et al., 2013; Henning et al., 2014).
The Aim of the Present Written report
The aim of this study is an improved understanding of the patterns of diversity and endemism in these typical, diverse Andean plant taxa, based on actual latitudinal and altitudinal record data rather than assigning them to "units." The study addresses the following questions:
- Which latitudinal and altitudinal patterns of variety and endemism can be found in these mid-elevational taxa?
- Is at that place correspondence between current patterns of distribution and diversity with climate, uplift history, and topography?
- Is the AHZ a bulwark for north–s dispersal of mid-elevation taxa?
Materials and Methods
Species Data
The distribution data was collected during the terminal two decades working on several taxonomic papers. For the genus Nasa, our dataset, which was established during preparatory work for a revision in the Flora Neotropica, includes 1,151 records for all 128 taxa (98 species). Published data are taken from Weigend (1996, 2000a,b, 2001, 2004a,b, 2012b), Weigend et al. (1998, 2003), Dostert and Weigend (1999), Rodríguez and Weigend (1999, 2004, 2006), Weigend and Rodríguez (2001, 2002, 2003), Rodríguez et al. (2002), Henning and Weigend (2009a,b, 2011) and Henning et al. (2011), supplemented with some additional herbarium records. The information for Ribes are based on revised herbarium specimens and include 1,189 samples for the 56 species of Ribes subg. Parilla. Published distribution data are plant in Jørgensen and León-Yánez (1999), Weigend and Folder (2001a,b,c), Freire Fierro (2004), Weigend et al. (2005, 2010); Weigend and Rodríguez (2006), and Weigend (2008, 2012a). For the twenty species of "American Urtica," 592 samples are included in the dataset, based on Navas (1961), Soraru (1972), Weigend et al. (2005), and Weigend and Luebert (2009), unpublished specimen records.
Peculiarly many of the older specimens exercise non provide exact geographic coordinates. Thus, we had to georeference these based on the locality information stated on the herbarium labels with the assistance of spatial databases such every bit geonames.org, google earth, and google maps. Nosotros were able to assign coordinates with sufficient spatial resolution to i,038 out of the 1,151 specimens for Nasa, 994 out of 1,189 samples for Ribes, and 497 out of the 592 specimens for Urtica. For more than than ninety% of the specimens of Nasa and Urtica we were able to assign coordinates with an estimated fault of less than ±five km. For Ribes specimens, this is true for only ca. 60% of the specimens. However, the accuracy was high enough for all records for the mapping in 50 km × 50 km grid cells and 1° latitudinal bands (run into below). For the data on the distance, where the specimen was sampled, nosotros always used the original data stated on the herbarium label.
No phylogenies with species level resolution are available for the groups studied in this paper. The monophyly of Nasa was demonstrated by Weigend et al. (2004) and Weigend and Gottschling (2006). The phylogeny of the genus Ribes by Weigend et al. (2002) supports the independence and monophyly of Chilean and Argentinian sect. Parilla from the all tropical Andean species in the sect. Andina. The monophyly of "American Urtica" was demonstrated by Farag et al. (2013) and Henning et al. (2014).
Coding of life history/growth grade based on field observations (compare Figure 1) and cultivation data is directly forward for Urtica (annual herb, perennial herb, lianescent shrub) and Ribes (cushion plant, shrub, lianescent shrub), but more than problematical in Nasa. Nasa shows a broad range of growth forms, merely some of which are readily defined: biennial herbs, and perennial respectively deciduous shrubs, lianescent shrubs, stoloniferous versus rhizomatous perennial herbs (Weigend, 2001; Henning et al., 2011; compare Figures 1C–F). The categories annual and subperennial are more hard to distinguish: "imperceptible" annuals, such as Nasa urens (Effigy 1A) and North. chenopodiifolia are very curt-lived plants flowering for merely a few weeks and then passing into fruit. Larger annual species, such as N. olmosiana (Figure 1B), may flower for several months before finally dying afterwards producing fruits for several consecutive months. Other taxa, such as North. dilloniana, live for annihilation upwardly to a twelvemonth and—nether ideal conditions—decidedly longer. Unlike the taxa here defined as shrubs or perennial herbs, they lack whatever vegetative renewal growth, i.e., all lateral shoots immediately pass into flower, so that they are continuously in flower from reaching maturity and practice not tolerate any interruption of the growing flavour. This grouping of plants is therefore here classified every bit "subperennial" —they ultimately succumb to their ain weight or old historic period before reaching an age of ca. ii years and lack whatsoever ability for vegetative regeneration.

FIGURE one. Diverseness of life forms of the Andean Genera Nasa (Loasaceae, A–F), Ribes subgenus Parilla (Grossulariaceae, K–L), and Urtica (Urticaceae, Chiliad–P). (A) Nasa urens (annual), (B) N. olmosiana (annual), (C) Due north. weberbaueri (evergreen shrub), (D) N. magnifica (biennial), (E) N. ranunculifolia subsp. macrorrhiza (rhizomatous perennial herb), (F) North. sanagoranensis (deciduous shrub), (G) Ribes cuneifolium, (H) R. weberbaueri, (I) R. frankei, (J) R. viscosum, (K) R. hirtum, (L) R. macrobotrys, (Chiliad) Urtica longispica, (N) U. echinata, (O) U. leptophylla, (P) U. flabellata.
GIS Information
To clarify variety patterns of the three genera at dissimilar spatial scales we used equal-area grids (Behrmann projection) with 200 km × 200 km and 50 km × 50 km resolution (twoscore,000 and ii,500 km2 grid cells). Climate information was taken from the WORLDCLIM data gear up (Hijmans et al., 2005), a global data set with a spatial resolution of thirty", which equals ca. 1 km2 at the equator. To characterize species distributions, we used elevation data from the GTOPO30 dataset, which has xxx" spatial resolution, as well (U.Southward. Geological Survey, 1996). For the analyses of differences in slope along latitudinal and altitudinal gradients in the tropical Andes, we used the higher resolution SRTM Peak data with 90 m resolution (Jarvis et al., 2008), employing the Surface Analysis toolbox of the Spatial Analyst tools in ArcView. Mean gradient values and mean elevations are queried per 10 km × 10 km grid cells using the Zonal Statistics tools in ArcView.
Analyses
Data analyses and production of the graphics were performed in R Version 3.0.0 (R Development Core Squad, 2013). The threshold separating widespread and restricted range species were hither fix at 2° latitudinal range (ca. 220 km). Unfortunately, small scale spatial heterogeneity in the steep terrain of the Andes results in quality bug of the available climate datasets. Together with potential spatial errors of the georeferencing procedure this precluded more in-depth statistical analysis and modeling of multifariousness patterns for our written report with regard to fine-scale climate parameters. For the visualization of the distribution and diversity patterns on maps and for the overlay with environmental data, we used ArcView 9.3 (Environmental Systems Research Found [ESRI], 2008).
Results
Latitudinal and Altitudinal Patterns of Diversity
All three tropical groups studied here have a centre of diverseness in northern Republic of peru in the expanse called AHZ (ca. 3–8° South; Figures 2 and 3). This expanse is as well a center for restricted range species of all three genera. Looking at the detailed altitudinal and latitudinal diversity patterns in Figure 3, the highest diverseness of Ribes sect. Andina tin can be found slightly displaced due south and to college elevations.
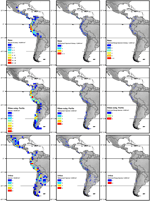
Figure 2. Overall species richness and richness of restricted range species (less than 2° latitudinal range) and more widespread species (more than 2° latitudinal range) of Nasa (top), Ribes (center), and Urtica (bottom). The diversity patterns are mapped in a 200 km × 200 km filigree (40,000 km2 grid cell size, maps on the left) respectively a l km × 50 km grid (2,500 km2 grid jail cell size) using Behrmann project. Topography is based on the GTOPO30 dataset (U.S. Geological Survey, 1996).

FIGURE iii. Latitudinal and altitudinal gradients of species richness and range size for Nasa (top), Ribes subg. Parilla (eye), and the "American Urtica" (bottom) in Latin America.
Nasa occurs in the tropical Andes from Venezuela to cardinal Republic of bolivia – just two taxa range into Primal America. Virtually species (73 out of 98) are endemic to Ecuador and Peru. Highest species diversity in the tropical Andes is plant at elevations of 2,500–3,500 m a.due south.fifty., smallest latitudinal ranges of species are observed between two,000 and four,500 m a.s.l. (Figure 3). In that location is a very articulate center of diversity at six° S with over twenty taxa in a 40,000 km2 grid prison cell (Figure 2). Here, the almost narrowly distributed taxa are found, with boilerplate latitudinal distributions of roughly 1–2°. Maximum species numbers are here found at all elevational levels between i,000 and 4,000 m a.s.l. Species across all altitudinal bands have small latitudinal ranges. High-summit species (mainly the N. ranunculifolia-group, compare Figure 4 and Henning et al., 2011) prove a slight south displacement, with the highest species numbers found effectually eight–13° S, clearly paralleling the displacement to higher elevations south of the AHZ in Ribes (Figure three). Overall, the taxa at the southern and especially the northern (13–24° N) distribution limit of the genus have comparatively wider latitudinal ranges. The highest morphological diversity with all known growth forms of the genus Nasa nowadays can exist found in the AHZ (Figure five). In add-on, species representing six of the viii growth forms have their median at more or less the same elevation around 3,000 m in the AHZ, showing a potent degree of altitudinal overlap. In the northern and primal Andes only subsets of the growth forms known from Nasa are reported and these bear witness moderate elevational segregation. Beyond the range, annual species are plant at lower elevations, with a slight displacement toward higher elevations in the central Andes (Figure 5). Latitudinal and altitudinal distribution of the nine informal infrageneric groups in Nasa as a proxy of phyletic diversity is shown in Effigy four. There is broad overlap of the unlike groups between 3 and 8° Southward, only one grouping is exclusively found exterior that region (N. venezuelensis-grouping in northern Colombia and Venezuela, Weigend, 1996). The highest species diversity is institute at 2,500–3,500 m, and the mean latitudinal range of the species consistently is less than 3° in the altitudinal bands to a higher place ii,000 m (Figure 6).
Effigy 4. Latitudinal and altitudinal distribution of the 11 infrageneric groups of the genus Nasa.
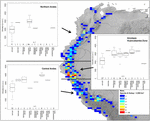
FIGURE 5. Elevational distribution of dissimilar life forms of Nasa compared for the northern Andes, the Amotape–Huancabamba zone, and the primal Andes. The topography in the map is based on the GTOPO30 dataset (U.S. Geological Survey, 1996). The elevation data for the assay is taken from the herbarium label data.

Figure 6. Total species richness and hateful latitudinal range per 500 grand altitudinal chugalug of all species of Nasa, Ribes sect. Andina, and the "American Urtica" in the northern Andes, the Amotape–Huancabamba Zone, and the fundamental Andes. The peak data for the analysis is taken from the original herbarium label data.
Ribes sect. Andina is nearly diverse in the northern and central Andes (10° North and xx° South, 43 species), including the AHZ. It has several peaks of variety along the latitudinal gradient (Figure 3: eight spp. per 1° latitudinal band) at one° S, 7° S, 9° Southward and 13° South, respectively. Species richness in 200 km × 200 km-filigree cells is highest in the AHZ in northern Peru (Figure 2) with upwards to 8 species co-occurring. Between six and 12° South there are particularly many taxa with relatively smaller ranges (Figure 2), whereas the southern distribution limit is represented by a single, widespread taxon (18–23° South). Of the 43 species, 35 are restricted to elevations above 2,000 m a.s.fifty., one-half of the species reach elevations of more than 4,000 chiliad, with the highest elevations reached between 10° S and 15° Due south. Highest diversity and smallest latitudinal ranges in the tropical Andes are found at elevations of 3,000–4,500 chiliad (Figures 3 and 6). Ribes sect. Parilla shows a contrasting pattern, with diversity peaking at middle elevations (ca. one,500 1000) around 37° S, but species beyond the altitudinal and latitudinal range are generally widespread. Across its range, mean range sizes of the species are roughly i club of magnitude larger than those of sect. Andina—while narrow endemics play a prominent role in sect. Andina, they are of subordinate importance in sect. Parilla.
Urtica lacks a clear center of diversity and the majority of species across the range are widely distributed. There are 2 peaks of both endemicity and diversity at 7–8° South and 16–17° South (Figures 2 and 3). The merely area with relatively narrowly distributed taxa (U. peruviana, U. lalibertadensis, U. urentivelutina) and a high overall species number is the AHZ around vii° Southward. In the southern function of the range (38–54° Due south) and the northern office of the South American range (6–11° N) only one widespread taxon is present (U. magellanica resp. U. leptophylla). Further north, in that location are 5 widespread species occurring mainly in United mexican states. Between 16° Due north and 21° S, Urtica is near restricted to elevations higher up 2,000 k. Highest species diversity in the tropical Andes is found at elevations of 2,500–3,500 thou, smallest latitudinal ranges are observed in species between ii,000 and 3,500 m (Figure 6). Both high and low elevation taxa are more widespread. Growth course diversity is low in Urtica, but all 3 growth forms recognized are present in the AHZ.
Is There Correspondence Between Electric current Patterns of Distribution and Diversity with Climate, Uplift History, and Topography?
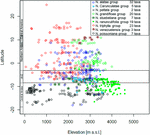
Climatic niches co-ordinate to overall wet and temperature plus seasonality of the two factors are shown in Figure 7. As mentioned in the methods department, the steep terrain of the Andes results in quality problems of the existing climate datasets which precluded more than in-depth statistical analysis of these patterns. Urtica is the most plastic of the groups, tolerating a wide range of overall humidity and temperature conditions plus a wide range of different degrees of seasonality. The frequent co-occurrence of Nasa, Urtica, and Ribes sect. Andina in nature is reflected by their overall similar ranges in climatic preference. All three cluster at depression temperature seasonality reflecting their high innertropical diversity. However, both Urtica and Nasa, to a much lesser degree Ribes sect. Andina, tolerate considerable seasonal variability in moisture. Ribes sect. Parilla is not segregated by overall temperature and moisture, just clearly segregates from sect. Andina in its decidedly higher accommodation to seasonal temperature variation. In comparison to Nasa and Urtica, Ribes sect. Parilla and sect. Andina have narrower climatic niches—separated by differences in seasonality.
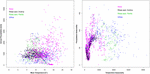
FIGURE 7. Climatic profiles of Nasa, Ribes sect. Andina, Ribes sect. Parilla, and the "American Urtica" in Latin America based on the WORLDCLIM data fix (Hijmans et al., 2005) with 30" spatial resolution. Precipitation seasonality is defined in the dataset as the coefficient of variation of the monthly precipitation, the temperature seasonality equally the standard departure of the monthly values x100.
The southern Andes (generally considered as the oldest office of the Andean chain) accept lower diversity and especially lower endemicity in the 2 groups distributed forth the unabridged Andean concatenation compared to the cardinal and northern Andes (Figures 2 and 3). Within the tropical Andes, the AHZ (the youngest role of the Andean concatenation) and the central Andes immediately South of the AHZ are institute to exist the areas of highest species richness and narrowest endemicity in all three groups under report. In Nasa this is partly due to the overlap of different infrageneric groups (Figure 4), which in plow correlate to different life histories (and ecological niches), which accordingly are most diverse in the AHZ (Figure v). A parallel, but less pronounced, situation is found in Ribes and Urtica.
The distribution of slope inclination for five areas along the tropical Andes clearly shows that the steepest slopes are nearly common at elevations of 2,000–3,500 m a.due south.l., roughly respective to the peaks of species diversity of the groups under study (Figure 8). Only in the Andes of primal Peru (ten–11° S) there is a 2nd maximum at approximately 4,500–5,000 1000.
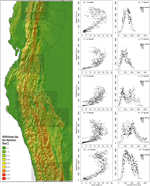
Effigy viii. Mean gradient in relation to altitude in five Due east-West-Transects (each 1° North–South) in the tropical Andes. Mean elevation and mean slope per 10 km × 10 km grid cell was computed based on SRTM Elevational data with 90 m spatial resolution (Jarvis et al., 2008).
Is the Amotape–Huancabamba Zone a Barrier for N–South Dispersal of Mid-Elevation Taxa?
The southern and northern distribution limits for the species in the groups under investigation are summarized in Figure ix. The graphics for overall species numbers identify no region where particularly many species achieve their northern or southern limits in any of the groups. If all species are considered then at that place seems to be a particular "border" in the AHZ for Nasa (5–viii° S), where many species reach their southern or northern limit. This is, notwithstanding, entirely an artifact of the abundance of narrowly endemic taxa in this region. Equally soon as the analysis is restricted to the taxa with a latitudinal distribution of more than than ii°, the pattern vanishes and species more than or less randomly achieve their limits betwixt five° N and xviii° S. Pooling data on all 67 of these "widespread" taxa in the establish groups studied, the southern limit of the tropical Andes in Bolivia (ca. 17° Due south) comes out every bit a adequately clear southern distribution limit for 6 of the taxa (less than 10% of the "widespread" taxa), and at that place is also a southern distribution limit for the same number of taxa simply north of the AHZ. However, no part of the tropical Andes is recognized every bit an overall important distribution limit for a major number of taxa.
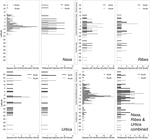
FIGURE 9. Latitudinal positions of northern and southern range boundaries for all species of Nasa, Ribes subg. Parilla, and Urtica compared to the patters of the more widespread species with at least 2° latitudinal range (ca. 220 km).
Give-and-take
Overall latitudinal diversity patterns plant here roughly show at least for Nasa and Ribes more than widely distributed species toward the distribution limits of the individual groups and more narrowly distributed taxa virtually the equator (compare Rohde, 1992, 1999). The actual peak of diversity and restricted range taxa is displaced clearly from the equator to ca. three–8° S. Patterns of endemicity and both altitudinal and latitudinal multifariousness of the three tropical Andean groups studied are remarkably similar, with Nasa, Ribes, and Urtica having peaks of diversity in the AHZ. The patterns for Ribes and Urtica show a deportation into higher elevations in the tropics compared to higher latitudes, as would be expected from primarily temperate plant groups. The elevational diversity patterns of the tropical Andean herb and shrub species investigated in this study evidence a acme at relatively high elevations of ca. 2,500–3,500 one thousand. Latitudinal range of highest elevation taxa is not notably different from that of lower elevational bands. As shown by Braun et al. (2002) the upper wood line in the Andes reaches highest elevation at ca. ten° S of the equator, and this is roughly the region where Ribes and the high Andean Due north. ranunculifolia-group have their centers of diverseness. This region would hither technically belong to the northernmost part of the fundamental Andes. Both the elevational band with highest species numbers and the southward displacement of maximum diversity are parallel to the patterns reported for Puya by Jabaily and Sytsma (2013). Previous studies, based on all angiosperms, mostly found a diversity acme at lower elevations (Kessler, 2001; Braun et al., 2002; Van Der Werff and Consiglio, 2004; Jørgensen et al., 2011), which is to be expected since they all included lowland taxa.
The bulk of the herb and shrub species studied hither are found at disturbed sites and correspond early on (almanac, biennial, subperennial Nasa, Urtica) or mid- to late successional species (perennial Urtica, Ribes). Only a few of the forest species are institute in climax vegetation. The peak of species variety parallels the elevation band with the occurrence of the steepest slopes in the Andes (Figure 8). Different landslide chance assessments found that gradient (inclination) is amongst the most of import parameters to predict natural disturbance through landslides (e.chiliad., Dai and Lee, 2002; Ohlmacher and Davis, 2003; Goetz et al., 2011). For the mid-elevation forests (1,900–two,800 m a.s.l.) in the Carrasco National Park, Bolivia, information technology is estimated that 20% of the total tracheophyte flora depends on early successional vegetation types induced past landslides (Kessler, 1999). Landslides create new habitat islands, which are likely the driving force behind species survival, but too species isolation (founder event, eco-geographical isolation) in the taxa here studied. A crucial part of cloud wood dynamics due to landslides for creating and maintaining biodiversity has been repeatedly invoked (Kessler, 1999; Nöske et al., 2008; Restrepo et al., 2009; Richter et al., 2009) and the data here presented underscore this point for the taxa under study on a regional scale. Temporal dynamics of landscape heterogeneity is thus probable an important gene in Andean diversification, with different side by side habitat fragments representing unlike successional stages and housing different species complements under the same climatic and similar edaphic weather. The AHZ of southern Ecuador and northern Republic of peru likewise experiences the strongest influence of the El Niño Southern Oscillation (ENSO) resulting in subdecadal shifts in precipitation (Young, 2012), and this may add together an boosted aspect of temporal heterogeneity and dynamics, increasing diversity in this region.
One additional reason for differences in the levels of diversity can be deduced from the data on life history as proxy for ecological niches in the species studied: the zone with the highest species diversity also has the widest range of different growth forms, and the altitudinal differentiation of growth forms typical of other parts of the Andes is not realized (Figure 5): in the AHZ, typical "Puna" species (rhizomatous, perennial herbs) co-be with typical "subpáramo" species (evergreen shrubs) in Nasa in the aforementioned elevational band. This reflects the fact that in this part of the Andes small-scale differences in humidity and temperature lead to a habitat mosaic, permitting the co-existence of taxa with dramatically different climatic preferences in immediately neighboring, only climatically well differentiated habitat islands, often at like or identical elevations (Richter et al., 2009). The formation of a habitat mosaic, with individual forest fragments separated past more barren corridors is direct reflected in the presence of numerous micro-allopatric, just ecologically similar taxa, e.k., in Nasa (e.k., Dostert and Weigend, 1999; Henning and Weigend, 2009c). Analyzing diversity patterns of all ca. 1,400 species of Cacti, Barthlott et al. (in press) institute that the Andes of northern Peru (together with the Bolivian Andes) are also the most of import center of species with extremely modest distribution ranges in this plant grouping. In addition, the AHZ together with the Andes in southern Bolivia/northern Argentina is the region with the highest diversity at the level of Tribus. A report of Andean members of the genus Mimosa paint a similar picture with regard to diversification in the dry valleys of northern Peru and argues for the crucial function of topographic complexity in speciation in this region (Särkinen et al., 2011). Mimosa and Cacti are ecologically the diametrical opposite of the mesic groups studied here and it is thus at beginning highly surprising that they should too have a middle of endemicity in the aforementioned region. However, the caption is obvious: barren habitats are as much isolated past corridors of mesic habitats every bit conversely the mesic habitats are isolated from each other by dry out valleys and barren habitats. Both gross habitat types are thus nowadays in a highly fragmented mosaic landscape pattern and provide mutually complementary weather condition for micro-allopatric diversification.
It has been argued, that the geological history of the Andean concatenation is directly reflected in patterns of diversification and endemicity of Andean establish groups (Antonelli et al., 2009; Antonelli and Sanmartín, 2011). Nether this reasoning, high Andean taxa would exist expected to show elevated species numbers and loftier numbers of endemics in the oldest parts of the Andes, with a lower degree of multifariousness in younger parts of the Andean chain. In the iii tropical Andean groups under study, the contrary is true: the final part of the Andean chain to rise was the AHZ and hither we find the largest number of species, including endemics (and infrageneric groups in Nasa, indicating high phyletic diversity). Direct phylogenetic evidence for this zone being a centre of phyletic diverseness was also found by Struwe et al. (2009) in Macrocarpaea and past Smith and Baum (2006) in Iochrominae (Solanaceae), underscoring this counterintuitive finding. Recent publications, such as Cosacov et al. (2009) and Richter et al. (2009) persist in citing the actual Huancabamba Deflection (lowest pass: 5.v° South) as a dispersal barrier for Andean taxa. The analysis hither presented does non notice any generalized range limits either around 5.5° S or anywhere in the AHZ. Smith and Baum (2006), Cosacov et al. (2009), and Struwe et al. (2009) all bear witness that at that place were several dispersal events across the Huancabamba Deflection, and the data hither presented conspicuously evidence that this is a region of overlap between a range of different groups and a center of multifariousness, but that—based on the georeferenced data hither used—a general distribution limit or dispersal barrier in this region is not retrieved. Detailed specimen based studies, especially in high Andean plant groups, should therefore re-investigate this presumed barrier critically in the hereafter. Additionally, detailed phylogenetic investigations of a range of unlike plant groups, representing various habitats and elevational bands, could help to elucidate the historical processes of diversification in more detail.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed every bit a potential conflict of interest.
Acknowledgments
We want to limited our sincere gratitude to the various colleagues who assisted us during field studies, especially Eric Rodríguez Rodríguez (HUT, Trujillo, Republic of peru), Asunción Cano Echevarría (USM, Lima, Peru), Fatima Caceres Huamani (HUSA, Arequipa, Republic of peru). Holger Kreft and the three reviewers provided helpful suggestions to improve this manuscript.
References
Antonelli, A., and Sanmartín, I. (2011). Mass extinction, gradual cooling, or rapid radiations? Reconstructing the spatiotemporal evolution of the ancient angiosperm genus Hedyosmum (Chloranthaceae) using empirical and simulated approaches. Syst. Biol. 60, 596–615. doi: ten.1093/sysbio/syr062
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Barthlott, Westward., Burstedde, K., Geffert, J. L., Ibisch, P. L., Korotkova, North., Miebach, A.,et al. (in printing). Biogeography and biodiversity of Cacti. Schumannia.
Google Scholar
Barthlott, W., Mutke, J., Rafiqpoor, Thousand. D., Kier, One thousand., and Kreft, H. (2005). Global centres of vascular plant diversity. Nova Acta Leopold. 92, 61–83.
Google Scholar
Bell, C. D., and Donoghue, M. J. (2005). Phylogeny and biogeography of Valerianaceae (Dipsacales) with special reference to the South American valerians. Org. Divers. Evol. 5, 147–159. doi: ten.1016/j.ode.2004.ten.014
CrossRef Total Text | Google Scholar
Braun, G., Mutke, J., Reder, A., and Barthlott, W. (2002). "Biotope patterns, phytodiversity and forestline in the Andes, based on GIS and remote sensing data" in Mountain Biodiversity: A Global Assessment, eds C. Körner and Eastward. One thousand. Spehn (London: Parthenon Publishing),75–89.
Google Scholar
Cosacov, A., Sérsic, A. N., Sosa, V., De-Nova, J. A., Nylinder, S., and Cocucci, A. A. (2009). New insights into the phylogenetic relationships, graphic symbol evolution, and phytogeographic patterns of Calceolaria (Calceolariaceae). Am. J. Bot. 96, 2240–2255. doi: 10.3732/ajb.0900165
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text | Google Scholar
Dai, F. C., and Lee, C. F. (2002). Landslide characteristics and slope instability modeling using GIS, Lantau Island, Hong Kong. Geomorphology 42, 213–228. doi: 10.1016/S0169-555X(01)00087-3
CrossRef Total Text | Google Scholar
Dostert, North., and Weigend, M. (1999). A synopsis of the Nasa triphylla circuitous (Loasaceae), including some new species and subspecies. Harv. Pap. Bot. iv, 439–467.
Google Scholar
Emadzade, K., Lehnebach, C., Lockhart, P., and Hörandl, Due east. (2010). A molecular phylogeny, morphology and classification of genera of Ranunculeae (Ranunculaceae). Taxon 59, 809–828.
Pubmed Abstract | Pubmed Full Text | Google Scholar
Ecology Systems Inquiry Found [ESRI]. (2008). ArcMAP 9.three. Redlands, CA: Environmental Systems Research Institute.
Google Scholar
Farag, M. A., Weigend, M., Luebert, F., Brokamp, G., and Wessjohann, L. A. (2013). Phytochemical, phylogenetic, and anti-inflammatory evaluation of 43 Urtica accessions (stinging nettle) based on UPLC–Q-TOF-MS metabolomic profiles. Phytochemistry 96, 170–183. doi: 10.1016/j.phytochem.2013.09.016
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text | Google Scholar
Freire Fierro, A. (2004). "Grossulariaceae," in Flora of Ecuador, Vol. 73, eds G. Harling and L. Andersson(Göteborg: University of Göteborg), 41–66.
Google Scholar
Gentry, A. H. (1982). Neotropical floristic diversity: phytogeographical connections betwixt fundamental and South America, pleistocene climatic fluctuations, or an accident of the Andean orogeny? Ann. Mo. Bot. Gard. 69, 557–593. doi: 10.2307/2399084
CrossRef Full Text | Google Scholar
Goetz, J. N., Guthrie, R. H., and Brenning, A. (2011). Integrating physical and empirical landslide susceptibility models using generalized additive models. Geomorphology 129, 376–386. doi: 10.1016/j.geomorph.2011.03.001
CrossRef Full Text | Google Scholar
Henning, T., Quandt, D., Grosse-Veldmann, B., Monro, A., and Weigend, M. (2014). Weeding the Nettles Ii: a delimitation of "Urtica dioica 50."(Urticaceae) based on morphological and molecular data, including a rehabilitation of Urtica gracilis Ait. Phytotaxa 162, 61–83. doi: 10.11646/phytotaxa.162.ii.1
CrossRef Full Text | Google Scholar
Henning, T., Rodríguez, Due east. F., and Weigend, M. (2011). A revision of the Nasa ranunculifolia group (Nasa ser. Grandiflorae pro parte, Loasaceae). Bot. J. Linn. Soc. 167, 47–93. doi: x.1111/j.1095-8339.2011.01164.ten
CrossRef Full Text | Google Scholar
Henning, T., and Weigend, M. (2009a). Nasa sanagoranensis spec. nov.—a new shrubby species of Nasa Weigend ser. Carunculatae (Urb. & Gilg) Weigend (Loasaceae) from the Amotape-Huancabamba Zone. Rev. Peru. Biol. 16, 151–156.
Google Scholar
Henning, T., and Weigend, M. (2009b). Systematics of the Nasa poissoniana group (Loasaceae) from Andean South America. Bot. J. Linn. Soc. 161, 278–301. doi: 10.1111/j.1095-8339.2009.01006.x
CrossRef Full Text | Google Scholar
Henning, T., and Weigend, M. (2009c). 2 novel and critically endangered subspecies of Nasa humboldtiana (Loasaceae) from Peru. Bot. Jahrb. 127, 473–488. doi: 10.1127/0006-8152/2009/0127-0473
CrossRef Total Text | Google Scholar
Henning, T., and Weigend, Thou. (2011). Ii new species of Nasa (Loasaceae) from Andean S America. Phytotaxa 26, 1–8.
Google Scholar
Hershkovitz, M. A., Arroyo, M. T. K., Bong, C., and Hinojosa, L. F. (2006). Phylogeny of Chaetanthera (Asteraceae: Mutisieae) reveals both ancient and contempo origins of the loftier elevation lineages. Mol. Phylogenet. Evol. 41, 594–605. doi: 10.1016/j.ympev.2006.05.003
Pubmed Abstruse | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hijmans, R. J., Cameron, S. E., Parra, J. Fifty., Jones, P. M., and Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. doi: 10.1002/joc.1276
CrossRef Full Text | Google Scholar
Hoorn, C., Wesselingh, F. P., Ter Steege, H., Bermudez, Grand. A., Mora, A., Sevink, J.,et al. (2010). Amazonia through fourth dimension: Andean uplift, climate change, landscape development, and biodiversity. Science 330, 927–931. doi: 10.1126/scientific discipline.1194585
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ibisch, P. Fifty., Boegner, A., Nieder, J., and Barthlott, W. (1996). How various are neotropical epiphytes? An analysis based on the "Catalogue of flowering plants and gymnosperms of Peru." Ecotropica ii, thirteen–28.
Pubmed Abstruse | Pubmed Total Text | Google Scholar
Jabaily, R. S., and Sytsma, K. J. (2013). Historical biogeography and life-history evolution of Andean Puya (Bromeliaceae). Bot. J. Linn. Soc. 171, 201–224. doi: 10.1111/j.1095-8339.2012.01307.ten
CrossRef Full Text | Google Scholar
Janczewski, Eastward. (1907). Monographie de Groseillier. Mém. Soc. Phys. Sci. Nat. Genève 35, 199–517.
Google Scholar
Jarvis, A., Reuter, H. I., Nelson, A., and Guevara, Due east. (2008). Hole-Filled Seamless SRTM Data V4 [Online]. International Centre for Tropical Agriculture (CIAT). Available at: http://srtm.csi.cgiar.org [accessed July 27, 2013].
Google Scholar
Jørgensen, P. M., and León-Yánez, South. (eds). (1999). Catalogue of the Vascular Plants of Republic of ecuador. St. Louis, MI: Missouri Botanical Garden Press.
Google Scholar
Jørgensen, P. M., Ulloa Ulloa, C., León, B., León-Yánez, S., Brook, S. K., Nee, M.,et al. (2011). "Regional patterns of tracheophyte diversity and endemism," in Climate Modify and Biodiversity in the Tropical Andes, eds S. Chiliad. Herzog, R. Martinez, P. K. Jørgensen, and H. Tiesse [São José dos Campos: Inter-American Establish for Global Alter Research (IAI) and Scientific Committee on Problems of the Environment (SCOPE)], 192–203.
Google Scholar
Kadereit, J. W., and von Hagen, One thousand. B. (2003). The development of flower morphology in gentianaceae-swertiinae and the roles of cardinal innovations and niche width for the diversification of Gentianella and Halenia in Due south America. Int. J. Plant Sci. 164, S441–S452. doi: 10.1086/376880
CrossRef Total Text | Google Scholar
Kessler, M. (1999). Constitute species richness and endemism during natural landslide succession in a perhumid montane forest in the Bolivian Andes. Ecotropica five, 123–136.
Google Scholar
Kessler, M. (2001). Patterns of diversity and range size of selected plant groups forth an elevational transect in the Bolivian Andes. Biodivers. Conserv. ten, 1897–1921. doi: x.1023/a:1013130902993
CrossRef Full Text | Google Scholar
Kessler, M. (2002). The elevational gradient of Andean plant endemism: varying influences of taxon-specific traits and topography at dissimilar taxonomic levels. J. Biogeogr. 29, 1159–1165. doi: x.1046/j.1365-2699.2002.00773.x
CrossRef Full Text | Google Scholar
Küper, W., Kreft, H., Nieder, J., Köster, N., and Barthlott, W. (2004). Large-scale diversity patterns of vascular epiphytes in Neotropical montane pelting forests. J. Biogeogr. 31, 1477–1487. doi: 10.1111/j.1365-2699.2004.01093.10
CrossRef Full Text | Google Scholar
Madriñán, South., Cortés, A. J., and Richardson, J. E. (2013). Páramo is the world's fastest evolving and coolest biodiversity hotspot. Front end. Genet. iv:192. doi: 10.3389/fgene.2013.00192
CrossRef Full Text | Google Scholar
Molau, U. (1988). Scrophulariaceae, I: Calceolarieae (Flora Neotropica Monograph No. 47). New York: The New York Botanical Garden Press.
Google Scholar
Mutke, J. (2011). "Biodiversity gradients," in Handbook of Biogeography, eds A. C. Millington, Grand. A. Blumler, M. Macdonald, and U. Schickhoff (London: Sage Publications), 168–188.
Google Scholar
Mutke, J., Kier, Thousand., Braun, G., Schultz, C., and Barthlott, West. (2001). Patterns of African tracheophyte diversity – a GIS based analysis. Syst. Geogr. Plants 71, 1125–1136. doi: ten.2307/3668744
CrossRef Full Text | Google Scholar
Mutke, J., Sommer, J. H., Kreft, H., Kier, G., and Barthlott, W. (2011). "Tracheophyte diversity in a changing world: global centres and biome-specific patterns.," in Biodiversity Hotspots, eds J. C. Habel and F. Zachos (Heidelberg: Springer), 83–96.
Google Scholar
Myers, Due north., Mittermeier, R. A., Mittermeier, C. One thousand., Da Fonseca, Thousand. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text | Google Scholar
Navas, E. (1961). El género Urtica en Republic of chile. Bol. Soc. Silverish. Bot. 9, 395–413.
Google Scholar
Nöske, Northward. Thousand., Hilt, N., Werner, F. A., Brehm, G., Fiedler, K., Sipman, H. J. M.,et al. (2008). Disturbance furnishings on diversity of epiphytes and moths in a montane forest in Ecuador. Basic Appl. Ecol. 9, 4–12. doi: x.1016/j.baae.2007.06.014
CrossRef Full Text | Google Scholar
Nürk, Due north. M., Madriñán, Southward., Carine, M. A., Hunt, M. W., and Blattner, F. R. (2013). Molecular phylogenetics and morphological evolution of St. John's wort (Hypericum; Hypericaceae). Mol. Phylogenet. Evol. 66, 1–16. doi: ten.1016/j.ympev.2012.08.022
Pubmed Abstract | Pubmed Full Text | CrossRef Total Text | Google Scholar
Ohlmacher, K. C., and Davis, J. C. (2003). Using multiple logistic regression and GIS technology to predict landslide chance in northeast Kansas, USA. Eng. Geol. 69, 331–343. doi: ten.1016/S0013-7952(03)00069-3
CrossRef Full Text | Google Scholar
Palazzesi, L., Barreda, V., and Tellería, Yard. C. (2009). Fossil pollen grains of Sunflower family from the Miocene of Patagonia: Barnadesioideae affinity. Rev. Palaeobot. Palynol. 155, 83–88. doi: 10.1016/j.revpalbo.2009.03.001
CrossRef Full Text | Google Scholar
Palazzesi, L., Gottschling, K., Barreda, V., and Weigend, Thou. (2012). Start Miocene fossils of Vivianiaceae shed new lite on phylogeny, divergence times, and historical biogeography of Geraniales. Biol. J. Linn. Soc. 107, 67–85. doi: 10.1111/j.1095-8312.2012.01910.10
CrossRef Full Text | Google Scholar
Prance, G. T. (1989). "American tropical forests," in Ecosystems of the World 14B: Tropical Rain Forest Ecosystems, eds H. Lieth and M. J. A. Werger (Amsterdam: Elsevier), 99–132.
Google Scholar
R Development Core Team. (2013). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. Available at: http://www.R-projection.org
Google Scholar
Restrepo, C., Walker, L. R., Shiels, A. B., Bussmann, R., Claessens, L., Fisch, S.,et al. (2009). Landsliding and its multiscale influence on mountainscapes. Bioscience 59, 685–698. doi: x.1525/bio.2009.59.8.10
CrossRef Full Text | Google Scholar
Richter, M., Diertl, K.-H., Emck, P., Peters, T., and Beck, E. (2009). Reasons for an outstanding plant multifariousness in the tropical Andes of Southern Ecuador. Landsc. Online 12, 1–35. doi: 10.3097/LO.200912
CrossRef Full Text | Google Scholar
Rodríguez, East. F., and Weigend, 1000. (1999). Nasa umbraculifera (Loasaceae: Loasoideae), una nueva especies con hojas peltadas para el Perú. Arnaldoa vi, 49–56.
Google Scholar
Rodríguez, E. F., and Weigend, M. (2004). Nasa longivalvis (Loasaceae: Loasoideae), una nueva especie del Departamento de La Libertad, Perú. Arnaldoa xi, 67–78.
Google Scholar
Rodríguez, E. F., Weigend, Thou., and Dostert, N. (2002). Sobre la validez de Nasa dyeri subsp. dyeri (Loasaceae) como united nations nuevo reporte para la flora peruana. Arnaldoa nine, 21–25.
Google Scholar
Rohde, Thousand. (1992). Latitudinal gradients in species multifariousness: the search for the primary crusade. Oikos 65, 514–527. doi: 10.2307/3545569
CrossRef Full Text | Google Scholar
Rohde, G. (1999). Latitudinal gradients in species diversity and Rapoport'south dominion revisited: a review of recent work and what tin parasites teach us nigh the causes of the gradients? Ecography 22, 593–613. doi: 10.1111/j.1600-0587.1999.tb00509.x
CrossRef Full Text | Google Scholar
Särkinen, T. E., Marcelo-Peña, J. L., Yomona, A. D., Simon, M. F., Pennington, R. T., and Hughes, C. East. (2011). Underestimated endemic species diversity in the dry inter-Andean valley of the Río Marañón, northern Peru: an instance from Mimosa (Leguminosae, Mimosoideae). Taxon 60, 139–150.
Google Scholar
Soraru, Due south. B. (1972). Revision de las Urticaceae argentinas. Darwiniana 17, 258–279.
Google Scholar
Struwe, L., Haag, S., Heiberg, Eastward., and Grant, J. R. (2009). Andean speciation and vicariance in neotropical Macrocarpaea (Gentianaceae-Helieae). Ann. Mo. Bot. Gard. 96, 450–469. doi: 10.3417/2008040
CrossRef Full Text | Google Scholar
Van Der Werff, H., and Consiglio, T. (2004). Distribution and conservation significance of endemic species of flowering plants in Peru. Biodivers. Conserv. 13, 1699–1713. doi: x.1023/B:BIOC.0000029334.69717.f0
CrossRef Full Text | Google Scholar
von Hagen, K. B., and Kadereit, J. West. (2001). The phylogeny of Gentianella (Gentianaceae) and its colonization of the southern hemisphere as revealed by nuclear and chloroplast Dna sequence variation. Org. Divers. Evol. 1, 61–79. doi: ten.1078/1439-6092-00005
CrossRef Full Text | Google Scholar
Vuilleumier, F. (1968). Population structure of the Asthenes flammulata superspecies (Aves: Fumariidae). Breviora 297, ane–32.
Google Scholar
Weddell, H. A. (1856). Monographie de la famille des urticacées. Nouv. Arch. Mus. Hist. Nat. Paris 9, 1–592.
Google Scholar
Weddell, H. A. (1869). "Urtica," in Prodromus Systematis Naturalis Regni Vegetabilis, ed. A. D. De Candolle (Paris: Treuttel and Würtz), 39–67.
Google Scholar
Weigend, 1000. (1996). Notes on Loasa II: "Cajophora" venezuelensis Steyerm. and its allies. Sendtnera three, 232–236.
Google Scholar
Weigend, K. (2000a). "132. Loasaceae," in Flora of Ecuador, Vol. 64, eds L. Andersson and G. Harling (Göteborg: University of Göteborg), 1–92.
Google Scholar
Weigend, G. (2000b). A revision of the Peruvian species of Nasa ser. Alatae (Loasaceae). Nord. J. Bot. 20, 15–31. doi: 10.1111/j.1756-1051.2000.tb00727.x
CrossRef Full Text | Google Scholar
Weigend, M. (2001). "Loasaceae," in Flora De Republic of colombia, eds R. Bernal and E. Forero (Santa Atomic number 26 de Bogotá: Universidad Nacional de Republic of colombia), i–100.
Google Scholar
Weigend, M. (2003). "Loasaceae," in Flowering Plants of the Neotropics, eds N. Smith, South. A. Mori, A. Henderson, D. Due west. Stevenson, and Southward. V. Heald (Princeton, NJ: Princeton University Press), 217–219.
Google Scholar
Weigend, Grand. (2004a). Boosted observations on the biogeography of the Amotape-Huancabamba zone in northern Peru: defining the South-Eastern limits. Rev. Republic of peru. Biol. 11, 127–134.
Google Scholar
Weigend, 1000. (2004b). Four new species of Nasa ser. Alatae (Loasaceae) in the Amotape-Huancabamba zone of Peru. Novon xiv, 134–146.
Google Scholar
Weigend, M. (2007). "Grossulariaceae," in The Families and Genera of the Vascular Plants, ed. K. Kubitzki (Berlin: Springer), 168–176.
Google Scholar
Weigend, Thousand. (2008). "Grossulariaceae," in Catálogo de las Plantas Vasculares del Cono Sur (Argentine republic, sur de Brasil, Chile, Paraguay y Uruguay), eds F. O. Zuloaga, O. Morrone, and M. J. Belgrano, 2348.
Google Scholar
Weigend, M. (2012a). "Grossulariaceae," in Flora de Antioquia: Catálogo de las Plantas Vasculares, Vol. II, Listado de las Plantas Vasculares del Departamento de Antioquia, eds A. Idárraga, R. Del, C. Ortiz, R. Callejas, and M. Merello (Bogotá: Universidad de Antioquia, Missouri Botanical Garden & Oficina de planeación departamental de la gobernación de Antioquia), 527.
Google Scholar
Weigend, Thou. (2012b). "Loasaceae," in Flora de Antioquia: Catálogo de las Plantas Vasculares, Vol. 2, Listado de las Plantas Vasculares del Departamento de Antioquia, eds A. Idárraga, R. D. C. Ortiz, R. Callejas, and Grand. Merello (Bogotá: Universidad de Antioquia, Missouri Botanical Garden & Oficina de Planeación Departamental de la Gobernación de Antioquia), 562.
Google Scholar
Weigend, M., and Binder, Yard. (2001a). A revision of the genus Ribes (Grossulariaceae) in Bolivia. Bot. Jahrb. Syst. Pflanzengeogr. 123, 111–134.
Google Scholar
Weigend, M., and Binder, M. (2001b). Ribes viscosum Ruiz & Pavon (Grossulariaceae), una especie ecológicamente importante de los Andes del Perú y su sinonimia. Arnaldoa 8, 39–44.
Google Scholar
Weigend, K., and Binder, M. (2001c). Three new species of Ribes L. (Grossulariaceae) from Cardinal and South America. Syst. Bot. 26, 727–732. doi: 10.1043/0363-6445-26.iv.727
CrossRef Full Text | Google Scholar
Weigend, Thou., Cano, A., and Rodríguez, E. F. (2005). New species and new records of the flora in Amotape-Huancabamba Zone: endemics and biogeographic limits. Rev. Peru. Biol. 12, 249–274.
Google Scholar
Weigend, Thousand., Cano, A., Rodríguez, E. F., and Breitkopf, H. (2010). Iv new species of Ribes (Grossulariaceae), primarily from the Amotape-Huancabamba Zone in northern Peru. Novon St. Louis Mo. 20, 228–238. doi: x.3417/2008090
CrossRef Full Text | Google Scholar
Weigend, M., Gottschling, M., Hoot, Southward., and Ackermann, 1000. (2004). A preliminary phylogeny of Loasaceae subfam. Loasoideae (Angiospermae: Cornales) based on trnL(UAA) sequence data, with consequences for systematics and historical biogeography. Org. Divers. Evol. 4, 73–ninety. doi: x.1016/j.ode.2003.12.001
CrossRef Full Text | Google Scholar
Weigend, One thousand., Henning, T., and Schneider, C. (2003). A revision of Nasa ser. Carunculatae (Loasaceae subfam. Loasoideae). Syst. Bot. 28, 765–781.
Google Scholar
Weigend, Chiliad., and Luebert, F. (2009). Weeding the nettles I: clarifying species limits in perennial, rhizomatous Urtica (Urticaceae) from southern and key Chile and Argentina. Phytotaxa two, 1–12. doi: 10.11646/phytotaxa.2.1.1
CrossRef Total Text | Google Scholar
Weigend, K., Mohr, O., and Motley, T. J. (2002). Phylogeny and nomenclature of the genus Ribes (Grossulariaceae) based on 5S-NTS sequences and morphological and anatomical data. Bot. Jahr. 124, 163–182. doi: ten.1127/0006-8152/2002/0124-0163
CrossRef Total Text | Google Scholar
Weigend, M., and Rodríguez, E. F. (2001). Nasa picta Hook. f. subsp. pamparomasii (Loasaceae), a new subspecies of Nasa picta from Ancash, Peru. Arnaldoa 7, 19–26.
Google Scholar
Weigend, M., and Rodríguez, E. F. (2002). Las espécies arbustivas de Nasa ser. Grandiflorae en el norte de Perú, con la descripción de una espécie nueva de la Abra de Barro Negro (Callacalla). Arnaldoa 9, 7–20.
Google Scholar
Weigend, 1000., and Rodríguez, E. F. (2003). A revision of the Nasa stuebeliana grouping [Nasa Weigend ser. Saccatae (Urb. & Gilg) Weigend p.p., Loasaceae] with notes on morphology, ecology, and distribution. Bot. Jahrb. 124, 345–382. doi: 10.1127/0006-8152/2003/0124-0345
CrossRef Total Text | Google Scholar
Weigend, One thousand., and Rodríguez, Eastward. F. (2006). Ribes amazonica spec. nov., la primera Ribes (Grossulariaceae) Peruana con inflorescencias erguidas. Arnaldoa 12, 42–47. doi: x.3417/2008090
CrossRef Full Text | Google Scholar
Weigend, Grand., Rodríguez, E. F., and Dostert, N. (1998). Nasa insignis y Nasa glandulosissima, dos especies nuevas de Nasa con hojas peltadas. Arnaldoa v, 151–157.
Google Scholar
Weigend, M., Zuloaga, F. O., Morrone, O., and Belgrano, Thou. J. (2008). "Grossulariaceae," in Catálogo de las Plantas Vasculares del Cono Sur (Argentina, sur de Brasil, Republic of chile, Paraguay y Uruguay), eds F. O. Zuloaga, O. Morrone, and M. J. Belgrano (St. Louis: Monographs in Systematic Botany from the Missouri Botanical Garden 107), 2348.
Google Scholar
Weir, J. T. (2009). Implications of genetic differentiation in Neotropical montane forest birds. Ann. Mo. Bot. Gard. 96, 410–433. doi: 10.3417/2008011
CrossRef Total Text | Google Scholar
Young, K. R. (2012). "Introduction to Andean geographies," in Climatic Change and Biodiversity in the Tropical Andes, eds S. Thousand. Herzog, R. Martinez, P. G. Jørgensen, and H. Tiessen (Chicago: MacArthur Foundation, IAI, Scope), 128–140.
Google Scholar
Young, Grand. R., and Reynel, C. (1997). "Huancabamba region, Peru and ecuador," in The Americas, Vol. 3, Centers of Plant Diversity, a Guide and Strategy for their Conservation, eds South. D. Davis, V. H. Heywood, O. Herrera-Macbryde, J. Villa-Lobos, and A. C. Hamilton (Cambridge: IUCN Publications Unit), 465–469.
Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fgene.2014.00351/full
0 Response to "what types of plants are important to the tropical andes"
Post a Comment